
On February 2, 2024, the Department of Health and Human Services (HHS), through the Substance Abuse and Mental Health Services Administration (SAMHSA), published a final rule that expands on, and makes permanent, certain opioid-related telehealth flexibilities initiated during the COVID-19 pandemic. Under the rule, authorized providers will be able to start patients on buprenorphine or methadone—medications used to treat opioid use disorders—pursuant to a telehealth visit and without needing an in-person visit.
At the onset of the COVID-19 pandemic, SAMHSA released guidance allowing for buprenorphine treatment to be initiated using telehealth, and allowing providers to dispense take-home doses of methadone based on their clinical judgment. Among other changes, the newly published final rule makes these flexibilities permanent.
The rule modifies provisions that govern Opioid Treatment Programs (OTPs) or practitioners registered with the DEA to dispense opioid agonist medication. Registered OTPs are the only programs through which people can access methadone treatment for opioid use disorders, and the new rule marks the first substantial change to treatment standards for OTPs in more than 20 years.
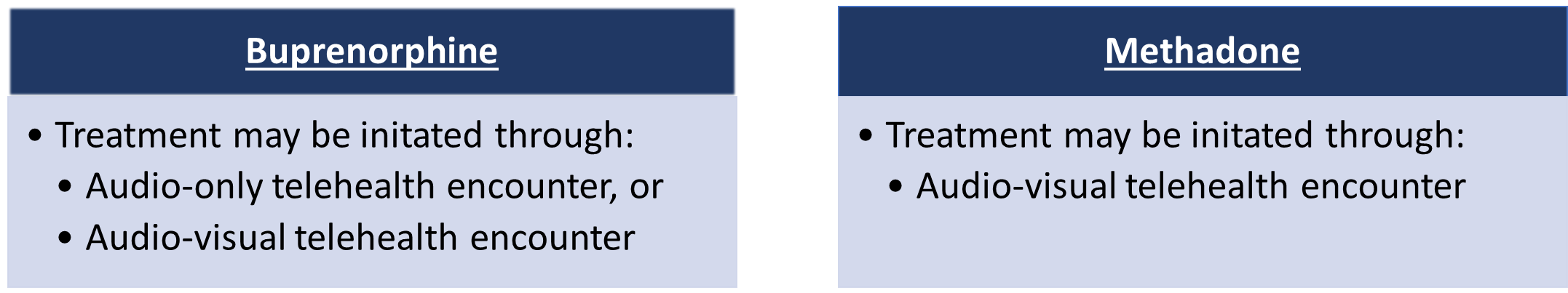
More specifically, the rule will permanently allow OTP physicians to initiate buprenorphine treatment for patients if they, or an authorized healthcare professional under an OTP physician’s supervision, determine that an adequate evaluation can be accomplished using audio-only or audio-visual telehealth technology. Similarly, the rule allows for OTP practitioners to initiate methadone treatment for patients if an adequate evaluation can be accomplished using audio-visual telehealth technology. The rule does not allow for the initiation of methadone treatment based on an audio-only telehealth encounter because, according to HHS, methadone has “a higher risk profile for sedation in patients presenting with mild somnolence,” which can be easier to identify in an audio-visual encounter.
The rule also only allows methadone to be ordered by an OTP practitioner pursuant to a telehealth visit, not to be prescribed for pick-up at a pharmacy. Methadone will still need to be dispensed at OTPs, though again providers will have more flexibility to dispense take-home doses to patients.
According to HHS, the final rule “makes treatment in OTPs more accessible to patients, while also supporting evidence-based and patient-centered care.” HHS pointed to recent research demonstrating that:
- Patients requiring treatment with buprenorphine have had high levels of satisfaction with telehealth services;
- The pandemic-related flexibilities were not associated with an increase in overdose deaths involving buprenorphine; and
- There have been no significant differences in treatment success and retention between patients who initiated buprenorphine via telehealth as opposed to in-person.
In addition to the flexibilities above, the rule also:
- Expands eligibility for patients to receive take-home doses of methadone;
- Expands access to interim treatment while awaiting further services;
- Updates accreditation and certification standards for OTPs;
- Expands the definition of “provider” to include more classes of healthcare professions who can order medications; and
- Aims to reduce stigmatizing language in SAMHSA regulations.
Lastly, while telehealth makes treatment more accessible for many people, some people may encounter challenges using telehealth technology. In the executive summary of the rule, HHS reminded healthcare providers that receive Federal financial assistance to ensure their telehealth platforms are accessible to individuals with disabilities and provide opportunity for meaningful access for individuals with limited English proficiency. HHS similarly reminded all providers of Federal civil rights laws that may require them to make reasonable accommodations to afford individuals with disabilities equal opportunity to benefit from treatment.
The new rule becomes effective April 2, 2024. To read the full text of the rule and an executive summary from HHS, go here.
Acknowledgements: Thank you to Tara Sklar, JD, MPH, Michael J. Holcomb, BS, Carrie Foote, BS, BA, and Mari Herreras, BA for editing and content recommendations.


